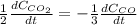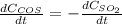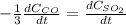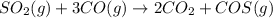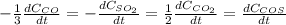Chemistry, 15.04.2020 17:39 tamariarodrigiez
Sulfur dioxide emissions in power plant stack gases may react with carbon monoxide as follows: SO_2 (g) + 3CO(g) rightarrow 2CO_2 + COS(g) Write an equation relating each of the following pairs of rates:
a. The rate of formation of CO_2 to the rate of consumption of CO
b. The rate of formation of COS to the rate of consumption of SO_2
c. The rate of consumption of CO to the rate of consumption of SO_2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
Sulfur dioxide emissions in power plant stack gases may react with carbon monoxide as follows: SO_2...
Questions in other subjects:

Mathematics, 28.10.2020 22:40


Mathematics, 28.10.2020 22:40

Mathematics, 28.10.2020 22:40

Mathematics, 28.10.2020 22:40



Chemistry, 28.10.2020 22:40

Mathematics, 28.10.2020 22:40