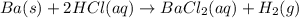Which of the following is an oxidation-reduction reaction? Which of the following is an oxidation-reduction reaction?
Pb(C2H3O2)2(aq) + 2 LiBr(aq) → PbBr2(s) + 2 LiC2H3O2(aq)
KI(aq) + AgNO3(aq) → AgI(s) + KNO3(aq)
HBr(aq) + KOH(aq) → KBr(aq) + H2O(l)
Ba(s) + 2 HCl(aq) → BaCl2(aq) + H2(g)
All of the above are oxidation-reduction reactions.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, krystenlitten
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
You know the right answer?
Which of the following is an oxidation-reduction reaction? Which of the following is an oxidation-re...
Questions in other subjects:






Medicine, 24.09.2020 16:01




Mathematics, 24.09.2020 16:01