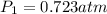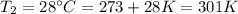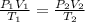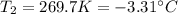Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3


Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
At what temperature in celsius would a gas have volume of 13.5 L at a pressure of 0.723 atm, if it h...
Questions in other subjects:

History, 27.06.2019 12:30

History, 27.06.2019 12:30



Social Studies, 27.06.2019 12:30


Biology, 27.06.2019 12:30

History, 27.06.2019 12:30