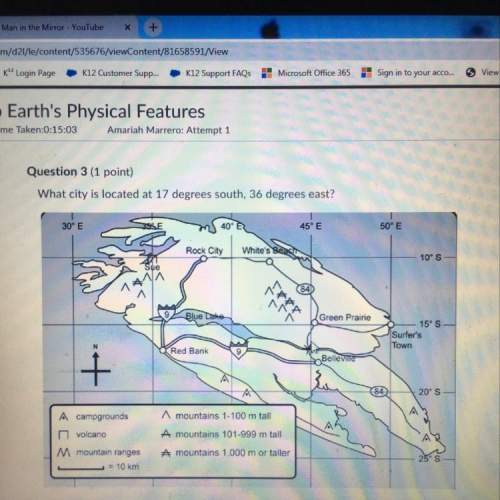
A concentration cell is constructed with copper electrodes and Cu2+ in each compartment. In one compartment, the [Cu2+] = 1.0 × 10–3 M and in the other compartment, the [Cu2+] = 2.0 M. Calculate the potential for this cell at 25°C. The standard reduction potential for Cu2+ is +0.34 V.
a. 0.78 V
b. –0.098 V
c. –0.44 V
d. 0.44 V
e. 0.098 V

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2


Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 03:30, cupcake3103670
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
A concentration cell is constructed with copper electrodes and Cu2+ in each compartment. In one comp...
Questions in other subjects:

English, 19.07.2019 07:50

English, 19.07.2019 07:50

English, 19.07.2019 07:50




English, 19.07.2019 07:50

English, 19.07.2019 07:50


English, 19.07.2019 07:50