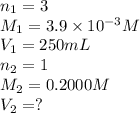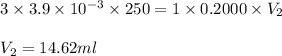Chemistry, 15.04.2020 15:52 crystalclear99
A chemistry student weighs out 0.09666 g of phosphoric acid (H3PO4), a triprotic acid, into a 250.volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.2000 M NaoH solution. Calculate the volume of NaoH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, baileysosmart
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3


Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 22.06.2019 18:30, ashleymer384
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
You know the right answer?
A chemistry student weighs out 0.09666 g of phosphoric acid (H3PO4), a triprotic acid, into a 250.vo...
Questions in other subjects:

Social Studies, 07.07.2019 22:20


Social Studies, 07.07.2019 22:20

Social Studies, 07.07.2019 22:20

Social Studies, 07.07.2019 22:20

Mathematics, 07.07.2019 22:20

Business, 07.07.2019 22:20

Biology, 07.07.2019 22:20

History, 07.07.2019 22:20

Computers and Technology, 07.07.2019 22:20

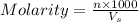
 = volume of solution in ml = 250 ml
= volume of solution in ml = 250 ml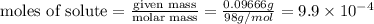
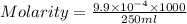
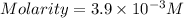
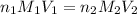
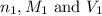 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 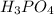
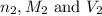 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.