
Chemistry, 15.04.2020 04:40 issagirl05
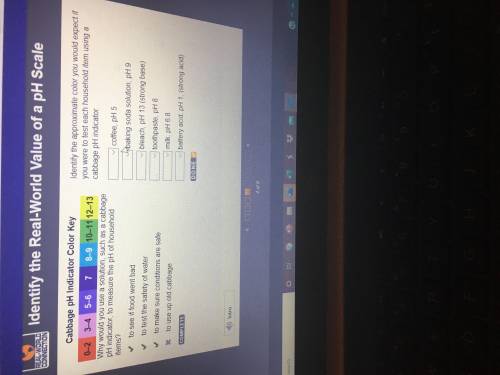
Identify the approximate color you would expect if you were to test each household item using a cabbage pH indicator.
Coffee, pH 5
Baking soda solution pH 9
Bleach, pH 13 (strong base)
Toothpaste, pH 6.8
milk, pH 6.8
Battery acid, pH 1 (strong acid)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, orlando19882000
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3


Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1
You know the right answer?
Identify the approximate color you would expect if you were to test each household item using a cabb...
Questions in other subjects:


History, 05.02.2020 02:48

Mathematics, 05.02.2020 02:48

Mathematics, 05.02.2020 02:48

Mathematics, 05.02.2020 02:48

History, 05.02.2020 02:48

Mathematics, 05.02.2020 02:48

Social Studies, 05.02.2020 02:48

History, 05.02.2020 02:48

Computers and Technology, 05.02.2020 02:48



