Chemistry, 15.04.2020 03:54 Ashley606hernandez
Diborane (B2H6) is a gas at room temperature that forms explosive mixtures with air. It reacts with oxygen according to the following equation (which may or may not be balanced): B2H6 (g) + O2 (g) → B2O3 (s) + H2O (l) How many grams of O2 (molar mass 32.00 g/mol) will react with 14.67 grams of diborane (molar mass 27.67 g/mol). Your answer must be expressed to the correct number of significant figures, and with the correct unit.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2



Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Diborane (B2H6) is a gas at room temperature that forms explosive mixtures with air. It reacts with...
Questions in other subjects:


Mathematics, 18.12.2019 05:31

Mathematics, 18.12.2019 05:31


Mathematics, 18.12.2019 05:31

Mathematics, 18.12.2019 05:31

Mathematics, 18.12.2019 05:31

Mathematics, 18.12.2019 05:31


Biology, 18.12.2019 05:31

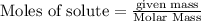
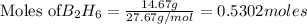
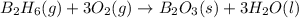
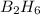 require = 3 moles of
require = 3 moles of 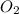
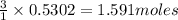 of
of