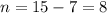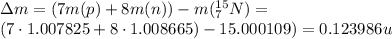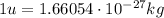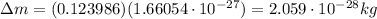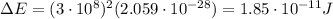Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, dustinquiz255
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 06:00, tytianadyson74
What volume of argon gas is equal to 1.60 grams of argon
Answers: 1
You know the right answer?
Calculate the binding energy per nucleon for a 157N715N nucleus. The mass of the neutral atom of 157...
Questions in other subjects:

Mathematics, 01.12.2020 23:30



Biology, 01.12.2020 23:30






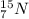 nucleus is 7.7 MeV. It was calculated with the mass of the neutral atom
nucleus is 7.7 MeV. It was calculated with the mass of the neutral atom 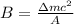
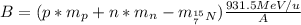 (1)
(1)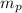 : is the proton's mass = 1.007825 u
: is the proton's mass = 1.007825 u 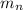 : is the neutron's mass = 1.008665 u
: is the neutron's mass = 1.008665 u 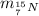 = 15.000109 u
= 15.000109 u 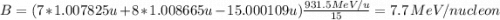
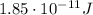
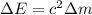 (1)
(1)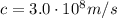 is the speed of light
is the speed of light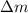 is the mass defect, which is the difference between the sum of the masses of the individual nucleons and the total mass of the nucleus.
is the mass defect, which is the difference between the sum of the masses of the individual nucleons and the total mass of the nucleus.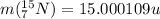
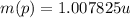 (mass of the proton)
(mass of the proton)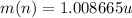 (mass of the neutron)
(mass of the neutron)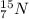 containes 7 protons (atomic number) and 15 nucleons (mass number), which means that the number of neutrons is
containes 7 protons (atomic number) and 15 nucleons (mass number), which means that the number of neutrons is