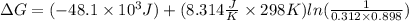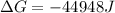Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1


Chemistry, 22.06.2019 18:00, meowmeowcow
Find the mass, in grams, of 5.00*10^23 molecules of f2
Answers: 3

Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
The value of ΔGo for the precipitation reaction,
Ca2+(aq) + CO32-(aq) <=> CaCO3(s) is -...
Ca2+(aq) + CO32-(aq) <=> CaCO3(s) is -...
Questions in other subjects:




History, 31.10.2020 01:20


Mathematics, 31.10.2020 01:20


Mathematics, 31.10.2020 01:20

Mathematics, 31.10.2020 01:20

Mathematics, 31.10.2020 01:20

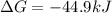
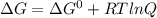
![Q=\frac{1}{[Ca^{2+}][CO_{3}^{2-}]}](/tpl/images/0601/0299/ddb27.png)