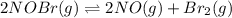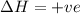Chemistry, 15.04.2020 03:13 joeykyle05
For the following reaction, the equilibrium constant Kc is 2.0 at a certain temperature. The reaction is endothermic. What do you expect to happen to the concentration of NO if the temperature is doubled? 2NOBr(g) ⇌ 2NO(g) + Br2(g)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 22:30, jaylenmiller437
The diagram shows the relationship between scientific disciplines. the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a. physics b. biology c. chemistry d. metallurgy
Answers: 2
You know the right answer?
For the following reaction, the equilibrium constant Kc is 2.0 at a certain temperature. The reactio...
Questions in other subjects:







Mathematics, 08.04.2021 20:50

Biology, 08.04.2021 20:50

Mathematics, 08.04.2021 20:50