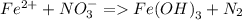Consider the following unbalanced redox reactions. In each case, separate the whole reactions into half-reactions, balance the half reactions, and combine to yield a balanced whole reaction. Compute the net reduction potential (E°) and pε° values for the net reactions and indicate whether the reaction is spontaneous as written. The reduction potential for Fe(OH)_3(s)/Fe^2+ is -0.181 V and pε is -3.08.
a. Fe^2 + + NO_3 = Fe(OH)_3(s) + N_2
b. Mn^2 + + Fe(OH)_3(s) = MnO_2(s) + Fe^2+

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3


Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
You know the right answer?
Consider the following unbalanced redox reactions. In each case, separate the whole reactions into h...
Questions in other subjects:

Geography, 02.03.2021 22:20

Mathematics, 02.03.2021 22:20

Mathematics, 02.03.2021 22:20


Mathematics, 02.03.2021 22:20

Mathematics, 02.03.2021 22:20

Mathematics, 02.03.2021 22:20

Mathematics, 02.03.2021 22:20

Biology, 02.03.2021 22:20

English, 02.03.2021 22:20