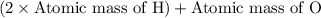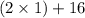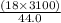Chemistry, 15.04.2020 03:07 nforrestall
A man ate 0.489 pound of cheese (an energy intake of 3.10 × 103 kJ). Suppose that none of the energy was stored in his body. What mass (in grams) of water would he need to perspire in order to maintain his original temperature? (It takes 44.0 kJ to vaporize 1 mole of water.) Enter your answer in scientific notation.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, pandasarecute53
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1


Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
A man ate 0.489 pound of cheese (an energy intake of 3.10 × 103 kJ). Suppose that none of the energy...
Questions in other subjects:


History, 26.10.2020 23:40

Advanced Placement (AP), 26.10.2020 23:40


Mathematics, 26.10.2020 23:40


Mathematics, 26.10.2020 23:40

Mathematics, 26.10.2020 23:40