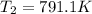Chemistry, 15.04.2020 02:57 christiannpettyy
The compound 1,1-difluoroethane decomposes at elevated temperatures to give fluoroethylene and hydrogen fluoride: CH3CHF2(g) → CH2CHF(g) + HF(g) At 460 °C, k = 5.8 × 10-6 s-1 and Ea = 265 kJ/mol. To what temperature (in K) would you have to raise the reaction to make it go four times as fast?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, goodygoodgirlygirl
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
The compound 1,1-difluoroethane decomposes at elevated temperatures to give fluoroethylene and hydro...
Questions in other subjects:



Mathematics, 07.11.2020 02:30



English, 07.11.2020 02:30


Spanish, 07.11.2020 02:30

Mathematics, 07.11.2020 02:30

Physics, 07.11.2020 02:30

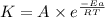
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0600/9306/6d953.png)
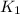 = rate constant at
= rate constant at 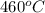 =
= 
 = rate constant at
= rate constant at 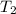 =
= 
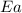 = activation energy for the reaction = 265 kJ/mol = 265000 J/mol
= activation energy for the reaction = 265 kJ/mol = 265000 J/mol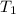 = initial temperature =
= initial temperature = 
![\log (\frac{4\times K_1}{K_1})=\frac{265000J/mol}{2.303\times 8.314J/mole.K}[\frac{1}{733K}-\frac{1}{T_2}]](/tpl/images/0600/9306/ddf14.png)