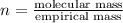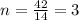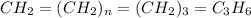Chemistry, 15.04.2020 02:55 bvbbridesmaid5519
Analysis of a sample of a gaseous compound shows that it contains 85.7% C and 14.3% H by mass. At standard conditions, 112 mL of the gaseous compound weighs 0.21 g. What is the molecular formula for the compound

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Analysis of a sample of a gaseous compound shows that it contains 85.7% C and 14.3% H by mass. At st...
Questions in other subjects:

Social Studies, 04.08.2019 02:00



Health, 04.08.2019 02:00

Chemistry, 04.08.2019 02:00


Mathematics, 04.08.2019 02:00

Social Studies, 04.08.2019 02:00

Business, 04.08.2019 02:00

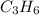
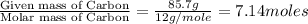
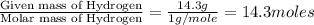
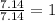
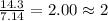
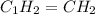
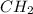 = 1(12) + 2(1) = 14 g/eq.
= 1(12) + 2(1) = 14 g/eq.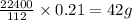 of compound
of compound