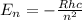Chemistry, 15.04.2020 03:00 tamekiablair502
What did Bohr conclude about the atom after observing emission spectrum lines a. Electrons can exist in any energy state but have specific states that are preferred. b. No two electrons can exist in the same quantum state within an atom. c. Possible electron energy states are quantized within an atom. d. Plum pudding tastes fantastic.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 23.06.2019 05:50, cicimarie2018
Which of the following is not a characteristic of s waves?
Answers: 1
You know the right answer?
What did Bohr conclude about the atom after observing emission spectrum lines a. Electrons can exist...
Questions in other subjects:


Spanish, 07.07.2019 05:00



Spanish, 07.07.2019 05:00


Spanish, 07.07.2019 05:00



English, 07.07.2019 05:00