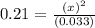Chemistry, 15.04.2020 02:43 prayingfeet7799
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium concentration of NO2(g). N2O4(g) ↔ 2 NO2(g) Kc = 0.21 [N2O4]eq = 0.033 M

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particula...
Questions in other subjects:




Mathematics, 14.10.2020 14:01



Physics, 14.10.2020 14:01



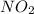 is 0.083 M
is 0.083 M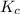
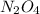 at equilibrium = 0.033 M
at equilibrium = 0.033 M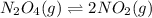
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0600/8631/271f5.png)