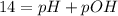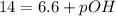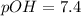Chemistry, 15.04.2020 00:57 sainijasdeep27
In pure water, some of the molecules ionize according to the equation H2O→H+ + OH−. The extent of the ionization increases with temperature. A student heats pure water and records the measured pH at 50°C as 6.6. Based on this information, what mathematical relationships gives the pOH pOH of pure water at 50°C?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, jasmineharris121
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
In pure water, some of the molecules ionize according to the equation H2O→H+ + OH−. The extent of th...
Questions in other subjects:

English, 24.10.2020 01:00

Computers and Technology, 24.10.2020 01:00





Mathematics, 24.10.2020 01:00

Biology, 24.10.2020 01:00


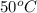 is,
is, 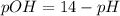
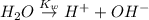
![K_w=[H^+][OH^-]](/tpl/images/0600/4070/bc68a.png)
![\log K_w=\log [H^+]+\log [OH^-]](/tpl/images/0600/4070/f0b12.png)
![-\log K_w=-\log [H^+]+(-\log [OH^-])](/tpl/images/0600/4070/00e23.png)
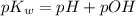
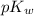 is 14 at 25-50°C.
is 14 at 25-50°C.