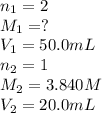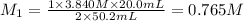Chemistry, 15.04.2020 01:00 dewillis9380
Carbonic acid, H2CO3, has two acidic hydrogens. A solution containing an unknown concentration of carbonic acid is titrated with potassium hydroxide. It requires 20.0 mL of 3.840 M KOH solution to titrate both acidic protons in 50.2 mL of the carbonic acid solution.
1. Write a balanced net ionic equation for the neutralization reaction. Include physical states.
2. Calculate the molarity of the carbonic acid solution.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, ayoismeisalex
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1


Chemistry, 22.06.2019 14:00, jivsf
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 23.06.2019 09:00, alisonlebron15
Are the results of a thoroughly tested hypothesis?
Answers: 2
You know the right answer?
Carbonic acid, H2CO3, has two acidic hydrogens. A solution containing an unknown concentration of ca...
Questions in other subjects:



Mathematics, 30.11.2020 21:20



Mathematics, 30.11.2020 21:20



History, 30.11.2020 21:20

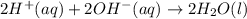
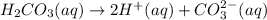 ..[1]
..[1]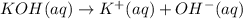 ..[2]
..[2]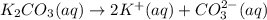 ..[3]
..[3]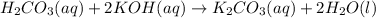
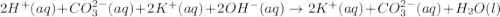
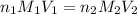
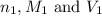 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 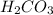
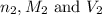 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.