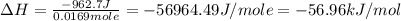In a coffee‑cup calorimeter, 65.0 mL of 0.890 M H 2 SO 4 was added to 65.0 mL of 0.260 M NaOH . The reaction caused the temperature of the solution to rise from 23.78 ∘ C to 25.55 ∘ C. If the solution has the same density as water (1.00 g/mL) and specific heat as water (4.184 J/g‑K), what is Δ H for this reaction (per mole of H 2 O produced)? Assume that the total volume is the sum of the individual volumes.

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 02:40, towelmearowel
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
In a coffee‑cup calorimeter, 65.0 mL of 0.890 M H 2 SO 4 was added to 65.0 mL of 0.260 M NaOH . The...
Questions in other subjects:


History, 04.02.2020 10:01

History, 04.02.2020 10:01

History, 04.02.2020 10:01



Mathematics, 04.02.2020 10:01

Physics, 04.02.2020 10:02


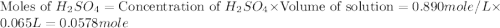
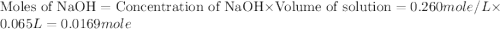
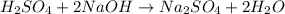
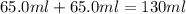
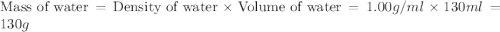
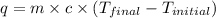
 = specific heat of water =
= specific heat of water = 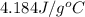
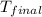 = final temperature of water =
= final temperature of water = 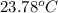
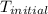 = initial temperature of metal =
= initial temperature of metal = 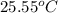
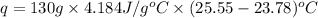
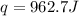
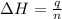
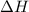 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?