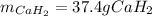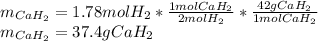Chemistry, 15.04.2020 00:28 swaggirllely36
Calcium hydride (CaH2) reacts with water to form hydrogen gas: CaH2(s) + 2 H2O(l) → Ca(OH)2(aq) + 2 H2(g) Determine the number of grams of CaH2 are needed to generate 55.0 L of H2 gas at a pressure of 0.811 atm and a temperature of 32°C.\

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:20, datboyjulio21
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 21.06.2019 23:20, anggar20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
Calcium hydride (CaH2) reacts with water to form hydrogen gas: CaH2(s) + 2 H2O(l) → Ca(OH)2(aq) + 2...
Questions in other subjects:


History, 20.10.2019 04:30

Mathematics, 20.10.2019 04:30

History, 20.10.2019 04:30


English, 20.10.2019 04:30

English, 20.10.2019 04:30

Chemistry, 20.10.2019 04:30


Biology, 20.10.2019 04:30