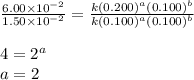Chemistry, 15.04.2020 01:53 beautycutieforever10
For the reaction 3A(g) + 2B(g) → 2C(g) + 2D(g) the following data were collected at constant temperature. Determine the correct rate law for this reaction. Trial Initial [A] Initial [B] Initial Rate (mol/L) (mol/L) (mol/(L·min)) 1 0.200 0.100 6.00 × 10–2 2 0.100 0.100 1.50 × 10–2 3 0.200 0.200 1.20 × 10–1 4 0.300 0.200 2.70 × 10–1

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 20:00, jalenevoyles
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
For the reaction 3A(g) + 2B(g) → 2C(g) + 2D(g) the following data were collected at constant tempera...
Questions in other subjects:


Mathematics, 12.09.2021 08:10

English, 12.09.2021 08:10

Mathematics, 12.09.2021 08:10


History, 12.09.2021 08:10




English, 12.09.2021 08:10

![\text{Rate}=k[A]^2[B]^1](/tpl/images/0600/6826/858f4.png)
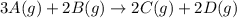
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0600/6826/10aeb.png)
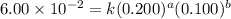 ....(1)
....(1)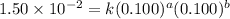 ....(2)
....(2)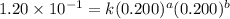 ....(3)
....(3)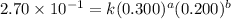 ....(4)
....(4)