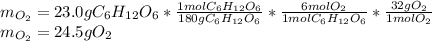Glucose, C6H12O6, is used as an energy source by the human body. The overall reaction in the body is described by the equation:
C6H12O6(aq) + 6O2(g) ⟶ 6CO2(g) + 6H2O(l)
1) Calculate the number of grams of oxygen required to convert 23.0 g of glucose to CO2 and H2O.
2) Calculate the number of grams of CO2 produced.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, sleimanabir
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 21.06.2019 22:00, applereams
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2


Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
Glucose, C6H12O6, is used as an energy source by the human body. The overall reaction in the body is...
Questions in other subjects:

Mathematics, 12.06.2020 02:57



English, 12.06.2020 02:57





Mathematics, 12.06.2020 02:57