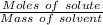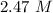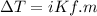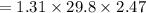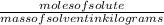Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, orlando19882000
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1


You know the right answer?
What is the freezing point of a solution made with 1.31 mol of CHCl3 in 530.0 g of CCl4 (Kf =29.8 de...
Questions in other subjects:










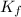 = 29.8° C/m
= 29.8° C/m