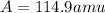Chemistry, 14.04.2020 22:30 alvarezreinier2
An element has two naturally-occurring isotopes. The mass numbers of these isotopes are 113 amu and 115 amu, with natural abundances of 5% and 95%, respectively. Calculate its average atomic mass. Report your answer to 1 decimal place.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3
You know the right answer?
An element has two naturally-occurring isotopes. The mass numbers of these isotopes are 113 amu and...
Questions in other subjects:



English, 30.10.2021 06:00

Computers and Technology, 30.10.2021 06:00


Mathematics, 30.10.2021 06:10


Biology, 30.10.2021 06:10

Mathematics, 30.10.2021 06:10

Mathematics, 30.10.2021 06:10

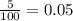
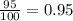
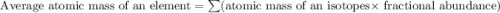
![A=\sum[(113\times 0.05)+(115\times 0.95)]](/tpl/images/0599/6688/a1f51.png)