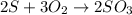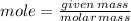Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, brittanygibson2812
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3


Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1
You know the right answer?
60.0 g O2 and 50.0 g S are reacted according to the equation 2 S + 3 O2 → 2 SO3 . Which reactant is...
Questions in other subjects:

History, 22.08.2021 14:00

Mathematics, 22.08.2021 14:00


Mathematics, 22.08.2021 14:00

Physics, 22.08.2021 14:00





English, 22.08.2021 14:00