
Chemistry, 14.04.2020 22:20 casting479
A sample of hydrogen gas occupies a volume of 6.38 L at 48.0°C and 0.490 atm. If it is desired to increase the volume of the gas sample to 8.10 L, while increasing its pressure to 0.655 atm, the temperature of the gas sample at the new volume and pressure must be °C.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 22:30, lori90
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 23.06.2019 00:30, cashkidd2200
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
You know the right answer?
A sample of hydrogen gas occupies a volume of 6.38 L at 48.0°C and 0.490 atm. If it is desired to in...
Questions in other subjects:

Mathematics, 05.03.2021 01:00








Mathematics, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00

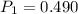 atm
atm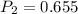 atm
atm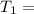 48° C = 321 K
48° C = 321 K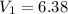 L
L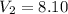 L
L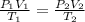
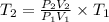
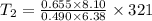
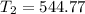 K
K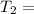 271.77°C
271.77°C

