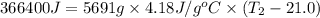Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
The balanced combustion reaction for C6H6 is 2C6H6(l)+15O2(g)⟶12CO2(g)+6H2O(l)+6 542 kJIf 8.700 g C6...
Questions in other subjects:

Mathematics, 26.08.2019 09:20

History, 26.08.2019 09:20

History, 26.08.2019 09:20

Mathematics, 26.08.2019 09:20

English, 26.08.2019 09:20

Mathematics, 26.08.2019 09:20


Mathematics, 26.08.2019 09:20


Mathematics, 26.08.2019 09:20


 = 8.700 g
= 8.700 g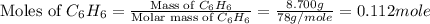



 = initial temperature =
= initial temperature = 
 = final temperature = ?
= final temperature = ?