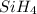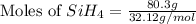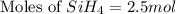Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 11:30, leapfroggiez
If a refrigerator is a heat pump that follows the first law of thermodynamics, how much heat was removed from food inside of the refrigerator if it released 300j of energy to the room?unit:
Answers: 1
You know the right answer?
The burning of 80.3 g of SiH4 at constant pressure gives off 3790 kJ of heat. Calculate △H for this...
Questions in other subjects:

Mathematics, 25.08.2020 09:01




Mathematics, 25.08.2020 09:01

Mathematics, 25.08.2020 09:01

Mathematics, 25.08.2020 09:01

Computers and Technology, 25.08.2020 09:01

History, 25.08.2020 09:01