
Chemistry, 14.04.2020 20:01 dontworry48
Consider the following reaction: 2CH2Cl2(g) CH4(g) CCl4(g) If 0.203 moles of CH2Cl2(g), 0.323 moles of CH4, and 0.240 moles of CCl4 are at equilibrium in a 15.2 L container at 477 K, the value of the equilibrium constant, Kc, is

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 00:30, StayPuftMarshadowMan
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
Consider the following reaction: 2CH2Cl2(g) CH4(g) CCl4(g) If 0.203 moles of CH2Cl2(g), 0.323 moles...
Questions in other subjects:

History, 02.06.2020 12:57

Mathematics, 02.06.2020 12:57

Social Studies, 02.06.2020 12:57

Chemistry, 02.06.2020 12:57

English, 02.06.2020 12:57

Mathematics, 02.06.2020 12:57

Mathematics, 02.06.2020 12:57

Mathematics, 02.06.2020 12:57

English, 02.06.2020 12:57

 at equilibrium= 0.203 mole
at equilibrium= 0.203 mole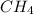 at equilibrium = 0.323 mole
at equilibrium = 0.323 mole at equilibrium = 0.240mole
at equilibrium = 0.240mole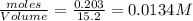
 =
= 
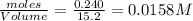

![K_c=\frac{[CH_4]\times [CCl_4]}{[CH_2Cl_2]^2}](/tpl/images/0599/0518/28fa5.png)




