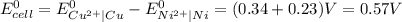Chemistry, 14.04.2020 18:32 lovemichelle638
Use standard reduction potentials to calculate the equilibrium constant for the reaction:
Cu²⁺ (aq) + Ni(s) → Cu(s) + Ni²⁺ (aq)
Hint: Carry at least 5 significant figures during intermediate calculations to avoid round off error when taking the antilogarithm.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

You know the right answer?
Use standard reduction potentials to calculate the equilibrium constant for the reaction:
Cu²...
Cu²...
Questions in other subjects:


Mathematics, 19.02.2021 19:10




Mathematics, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10


Social Studies, 19.02.2021 19:10


 ;
; 
 ;
;