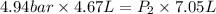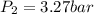Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
A certain mass of nitrogen gas occupies a volume of 4.67 L 4.67 L at a pressure of 4.94 bar 4.94 bar...
Questions in other subjects:


Geography, 13.10.2019 22:50

Mathematics, 13.10.2019 22:50


Physics, 13.10.2019 22:50

Mathematics, 13.10.2019 22:50




Biology, 13.10.2019 22:50

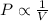
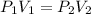
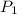 = initial pressure = 4.94 bar
= initial pressure = 4.94 bar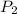 = final pressure = ?
= final pressure = ?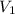 = initial volume = 4.67 L
= initial volume = 4.67 L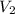 = final volume = 7.05 L
= final volume = 7.05 L