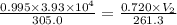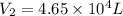Chemistry, 14.04.2020 17:50 jessica3981
A weather balloon is filled with helium that occupies a volume of 3.93 104 L at 0.995 atm and 32.0°C. After it is released, it rises to a location where the pressure is 0.720 atm and the temperature is -11.7°C. What is the volume of the balloon at that new location?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
A weather balloon is filled with helium that occupies a volume of 3.93 104 L at 0.995 atm and 32.0°C...
Questions in other subjects:

Biology, 30.06.2019 02:00

Mathematics, 30.06.2019 02:00


English, 30.06.2019 02:00


Mathematics, 30.06.2019 02:00

Mathematics, 30.06.2019 02:00

Mathematics, 30.06.2019 02:00


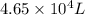
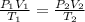
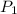 = initial pressure of gas = 0.995 atm
= initial pressure of gas = 0.995 atm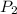 = final pressure of gas = 0.720 atm
= final pressure of gas = 0.720 atm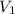 = initial volume of gas =
= initial volume of gas = 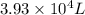
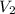 = final volume of gas = ?
= final volume of gas = ?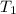 = initial temperature of gas =
= initial temperature of gas = 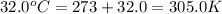
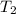 = final temperature of gas =
= final temperature of gas =