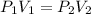Chemistry, 14.04.2020 16:33 lindasuebairdoyjpf7
A sample of an ideal gas at 1.00 atm and a volume of 1.87 L was placed in a weighted balloon and dropped into the ocean. As the sample descended, the water pressure compressed the balloon and reduced its volume. When the pressure had increased to 80.0 atm , what was the volume of the sample

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:00, vanessacox45
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 03:30, nikkio4
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus. b) the number of neutrons it contains in its nucleus. c) the number of protons it has in a cloud around the nucleus. d) the number of neutrons it has in a cloud around the nucleus. e) the number of electrons it exchanges with its neighbors.
Answers: 1

You know the right answer?
A sample of an ideal gas at 1.00 atm and a volume of 1.87 L was placed in a weighted balloon and dro...
Questions in other subjects:



Mathematics, 08.05.2021 17:30






Mathematics, 08.05.2021 17:30

 and
and 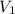 are the initial gas parameter before dropping into the ocean
are the initial gas parameter before dropping into the ocean and
and 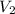 are the final gas parameter after dropping into the ocean
are the final gas parameter after dropping into the ocean