
Chemistry, 14.04.2020 16:12 buiratsamah
Butane (C4 H10(g), Hf = –125.6 kJ/mol) reacts with oxygen to produce carbon dioxide (CO2 , Hf = –393.5 kJ/mol ) and water (H2 O, Hf = –241.82 kJ/mol) according to the equation below. What is the enthalpy of combustion (per mole) of C4H10 (g)? Use .

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, 767sebmont
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2


Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
You know the right answer?
Butane (C4 H10(g), Hf = –125.6 kJ/mol) reacts with oxygen to produce carbon dioxide (CO2 , Hf = –393...
Questions in other subjects:

Geography, 26.06.2019 15:30




Mathematics, 26.06.2019 15:30


Mathematics, 26.06.2019 15:30


Mathematics, 26.06.2019 15:30

Social Studies, 26.06.2019 15:30

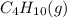 is -2657.5 kJ
is -2657.5 kJ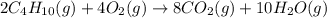
![\Delta H^o_{rxn}=[(8\times \Delta H^o_f_{CO_2(g)})+(10\times \Delta H^o_f_{H_2O(g)})]-[(1\times \Delta H^o_f_{C_4H_{10}(g)})+(4\times \Delta H^o_f_{O_2(g)})]](/tpl/images/0598/2199/66d33.png)
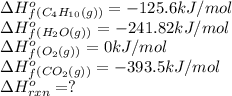
![\Delta H^o_{rxn}=[(8\times -393.5)+(10\times -241.82)]-[(2\times -125.6)+(4\times 0)]\\\\\Delta H^o_{rxn}=-5315kJ](/tpl/images/0598/2199/af1b6.png)


