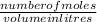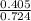Chemistry, 14.04.2020 07:44 aide1234564
1.Complete the balanced neutralization equation for the reaction below:
H2SO4 (aq) + Sr(OH)2 (aq) -->
2. How many L of a 0.209 M KI solution is needed to completely react with 2.43 g of Cu(NO3)2 according to the balanced chemical reaction:
2Cu(NO₃)₂(aq) + 4KI(aq) → 2CuI(aq) + I₂(s) + 4KNO₃(aq)
3.What volume in L of a 0.724 M Nal solution contains0.405 mol of Nal?
4. How many mL of 0.300 M NaF would be required to make a 0.0400 M solution of NaF when diluted to 250.0 mL with water?

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
1.Complete the balanced neutralization equation for the reaction below:
H2SO4 (aq) + Sr(OH)2 (...
H2SO4 (aq) + Sr(OH)2 (...
Questions in other subjects:

Mathematics, 03.07.2019 19:00

History, 03.07.2019 19:00


Mathematics, 03.07.2019 19:00

English, 03.07.2019 19:00


History, 03.07.2019 19:00

Mathematics, 03.07.2019 19:00


 S
S + Sr(OH)
+ Sr(OH) ⇒ SrSO4 + 2 H2O is the balanced reaction.
⇒ SrSO4 + 2 H2O is the balanced reaction.

 =
=