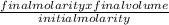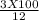Chemistry, 14.04.2020 06:12 jessicaa2350
In order to complete a lab, your teacher needs a 3.0 M solution of sulfuric acid, but only has a 12.0 M stock solution of sulfuric acid in the chemical store room. Calculate and describe the steps the teacher needs to take in order to make 100 mL of the 3.0 M solution of sulfuric acid.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1


Chemistry, 22.06.2019 17:30, kiaramccurty
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
In order to complete a lab, your teacher needs a 3.0 M solution of sulfuric acid, but only has a 12....
Questions in other subjects:


Chemistry, 17.07.2019 16:50

Advanced Placement (AP), 17.07.2019 16:50

Mathematics, 17.07.2019 16:50


History, 17.07.2019 16:50

History, 17.07.2019 16:50

English, 17.07.2019 16:50