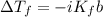Chemistry, 14.04.2020 01:51 TMeansStupidity
The normal freezing point of water is 0oC and it's freezing point depression constant is 1.86oC/m. If a 2.00 molal solution of Na2SO4 is prepared, what is the freezing point of the mixture?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 20:10, maddie1776
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
The normal freezing point of water is 0oC and it's freezing point depression constant is 1.86oC/m. I...
Questions in other subjects:


Mathematics, 20.10.2020 04:01


History, 20.10.2020 04:01

History, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01

English, 20.10.2020 04:01

Engineering, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01