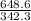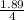Chemistry, 13.04.2020 22:49 20warriorsoul14
If 684.6g of sucrose (table sugar) are dissolved in 4 liters of water, what would be the molarity of the solution?
The chemical formula for sucrose is C12H22O11 , and the molar mass is 342.3 g/mol. mol/L

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
If 684.6g of sucrose (table sugar) are dissolved in 4 liters of water, what would be the molarity of...
Questions in other subjects:


History, 15.07.2019 13:00


Biology, 15.07.2019 13:00


Mathematics, 15.07.2019 13:00

Mathematics, 15.07.2019 13:00

History, 15.07.2019 13:00

Mathematics, 15.07.2019 13:00

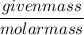
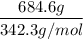
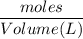

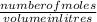 equation 1
equation 1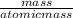 equation 2
equation 2