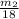Chemistry, 13.04.2020 22:36 AbigailHaylei
How many grams of water are produced when 4.50 L of
oxygen gas was consumed. The reaction temperature
and pressure are 425 K and 2.50 atm.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, marissastewart533
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
How many grams of water are produced when 4.50 L of
oxygen gas was consumed. The reaction temp...
oxygen gas was consumed. The reaction temp...
Questions in other subjects:

Mathematics, 14.05.2021 05:40


History, 14.05.2021 05:40


Mathematics, 14.05.2021 05:40

Mathematics, 14.05.2021 05:40

Social Studies, 14.05.2021 05:40



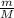
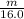 × 0.0821 × 425
× 0.0821 × 425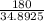
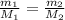
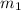 represents the mass of the oxygen
represents the mass of the oxygen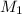 represents the gram molecular mass of the oxygen
represents the gram molecular mass of the oxygen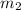 represents the mass of the water
represents the mass of the water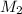 represents the gram molecular mass of water
represents the gram molecular mass of water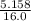 =
=