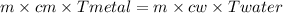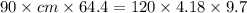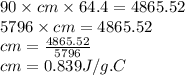Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2


Chemistry, 23.06.2019 17:00, bradenjesmt1028
An unknown substance was put in a container. the substance filled up the container from bottom up, taking half of the space in the container. what is most likely the state of matter of the substance? gas liquid plasma solid
Answers: 3
You know the right answer?
A 90.0 g piece of metal, initially at 98.6°C, is placed into 120.0 g of
water initially at 24....
water initially at 24....
Questions in other subjects:

Biology, 25.11.2020 01:00


English, 25.11.2020 01:00

English, 25.11.2020 01:00


Mathematics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

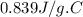 .
.