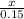Chemistry, 13.04.2020 18:33 gamerdoesart
A solution containing 20.0 g of sodium sulfite reacts with 7.0 ml of phosphoric acid. The concentration of the acid solution is such that there are 1.83 grams of H3PO4 per milliliter of solution. Determine the following: a. The mass of the excess reactant remaining at completion. b. Grams of water produced. c. Moles of sodium phosphate produced. d. Grams of sulfur dioxide produced.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, LarryJoeseph
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
A solution containing 20.0 g of sodium sulfite reacts with 7.0 ml of phosphoric acid. The concentrat...
Questions in other subjects:






Mathematics, 04.02.2021 18:40


Mathematics, 04.02.2021 18:40

Mathematics, 04.02.2021 18:40

 P
P + 3
+ 3 
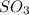 ⇒ 2
⇒ 2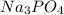 +3
+3 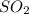 +3
+3 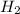 O
O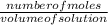
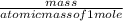
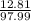
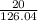
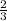 =
= 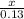
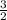 =
=