
Chemistry, 13.04.2020 08:26 DoomydoomGir
How much energy is needed to melt 161.0 g of ethanol(C₂H₅OH)?(Heat of fusion Ethanol- 5.02 KJ/mol)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:40, wbrandi118
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1


Chemistry, 21.06.2019 22:10, lilyjordan5972
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1
You know the right answer?
How much energy is needed to melt 161.0 g of ethanol(C₂H₅OH)?(Heat of fusion Ethanol- 5.02 KJ/mol)...
Questions in other subjects:










Health, 31.03.2020 02:37

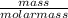
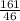 = 3.5mol
= 3.5mol

