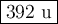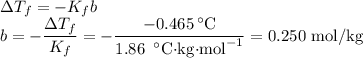Chemistry, 13.04.2020 01:29 Theresab2021
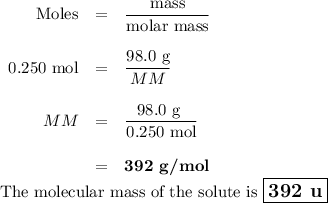
Suppose that 98.0g of a non electrolyte is dissolved in 1.00kg of water. The freezing point of this solution is found to be -0.465. What is the molecular mass of the solute?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mayamabjishovrvq9
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 21.06.2019 21:00, daigle18383
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2


You know the right answer?
Suppose that 98.0g of a non electrolyte is dissolved in 1.00kg of water. The freezing point of this...
Questions in other subjects:

Mathematics, 24.02.2022 08:30



Computers and Technology, 24.02.2022 08:30

Social Studies, 24.02.2022 08:30


Biology, 24.02.2022 08:30


Business, 24.02.2022 08:30