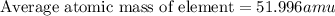Chemistry, 16.10.2019 03:00 22swittman
There are four naturally occurring isotopes of the element chromium. the relative abundance of each is 50cr - 4.345% 49.946044 amu 52cr - 83.789% 51.940508 amu 53cr - 9.501% 52.940649 amu 54cr - 2.365% 53.93880 amu find the average atomic mass of chromium. a. 52.061 amu b. 52.978 amu c. 51.996 amu d. 53.2503 amu

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1



Chemistry, 23.06.2019 18:30, choyontareq
You form water vapor by mixing oxygen and hydrogen at 730°c in a 5.4-liter container. this is the equation for the reaction: o2(g) + 2h2(g) → 2h2o(g). the partial pressure of oxygen before the reaction is 122.3 kilopascals, and there is excess hydrogen. how many moles of water are formed?
Answers: 3
You know the right answer?
There are four naturally occurring isotopes of the element chromium. the relative abundance of each...
Questions in other subjects:

Biology, 18.08.2019 01:10


Medicine, 18.08.2019 01:10







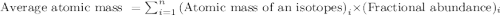
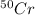 = 49.946044 amu
= 49.946044 amu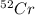 = 51.940508 amu
= 51.940508 amu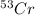 = 52.940649 amu
= 52.940649 amu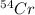 = 53.93880 amu
= 53.93880 amu![\text{Average atomic mass of element}=\sum[(49.946044\times 0.04345)+(51.940508\times 0.83789)+(52.940649\times 0.09501)+(53.93880\times 0.02365)]](/tpl/images/0323/8337/e3693.png)