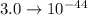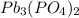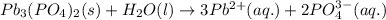The value of the Solubility Product Constant for lead phosphate is
Write the reaction t...

Chemistry, 11.04.2020 04:50 angelinaavila06
The value of the Solubility Product Constant for lead phosphate is
Write the reaction that corresponds to this Ksp value.
(Aq, S,L) +(Aq, S,L) <>(Aq, S,L) +(Aq, S,L)
Ksp values are found by clicking on the "Tables" link.
Use the pull-down menus to specify the state of each reactant or product.
If a box is not needed leave it blank.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 07:30, zamirareece17
1. list three scientific reasons cockroaches may fly.
Answers: 1

Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
Questions in other subjects:






Social Studies, 30.12.2020 23:30

Mathematics, 30.12.2020 23:30

Chemistry, 30.12.2020 23:30

English, 30.12.2020 23:30

Biology, 30.12.2020 23:30

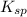 value of lead phosphate is given below and the value of solubility product for the same is
value of lead phosphate is given below and the value of solubility product for the same is