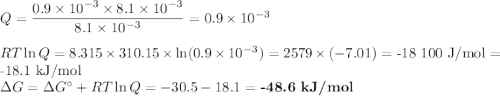Chemistry, 11.04.2020 01:50 ariellllllllllllllll
The equation for ATP hydrolysis is
ATP yields ADP + Pi delta G*= -30.5 kJ/mol
( H20)
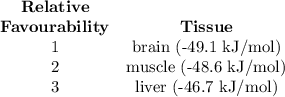
(a) Calculate ?G for ATP hydrolysis to rank the following conditions from most favorable to least favorable. Assume a temperature of 37.0C. R = 8.315 J/(mol. K).
ATP Hydrolysis most favorable
ATP hydroysis least favorable
The choices are:
a. muscle: [ATP]= 8.1mM; [ADP]= 0.9mM [Pi]= 8.1mM
b. brain: [ATP]= 2.6mM; [ADP]= 0.7mM [Pi]= 2.7mM
c. liver: [ATP]= 3.4mM; [ADP]= 1.3mM [Pi]= 4.8mM
(b) Calculate ?G for ATP hydrolysis in muscle at 18 degree C. Use the muscle concentrations from part a.
Delta g = kJ/mol

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
The equation for ATP hydrolysis is
ATP yields ADP + Pi delta G*= -30.5 kJ/mol
( H2...
ATP yields ADP + Pi delta G*= -30.5 kJ/mol
( H2...
Questions in other subjects:



History, 29.09.2021 21:00

Mathematics, 29.09.2021 21:00

Mathematics, 29.09.2021 21:00



Computers and Technology, 29.09.2021 21:00


Geography, 29.09.2021 21:00

![Q = \dfrac{\text{[ADP][P$_{\text{i}}$]}}{\text{[ADP]}}](/tpl/images/0594/8302/09bb9.png)