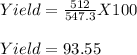Chemistry, 11.04.2020 00:00 clevelandjaniya
Use this balanced equation to help you solve:
1. If 842 grams of sodium hydroxide reacts with 750.0 grams of aluminum, how many grams of aluminum hydroxide should theoretically form?
2. If only 512 grams actually form, what was the % yield? (Briefly describe the steps you would take to solve).
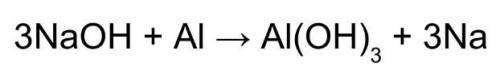

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1
You know the right answer?
Use this balanced equation to help you solve:
1. If 842 grams of sodium hydroxide reacts with...
1. If 842 grams of sodium hydroxide reacts with...
Questions in other subjects:


History, 10.06.2021 19:50

Mathematics, 10.06.2021 19:50

Mathematics, 10.06.2021 19:50


Mathematics, 10.06.2021 19:50

History, 10.06.2021 19:50


Mathematics, 10.06.2021 19:50

Mathematics, 10.06.2021 19:50

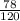 g of Al(OH)₃
g of Al(OH)₃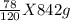 of Al(OH)₃
of Al(OH)₃