
Chemistry, 10.04.2020 21:57 tytybruce2
What is the activation energy of a reaction whose rate constant doubles when the temperature is increased from 300K to 350K (assuming that the activation energy and pre-exponential factor are both temperature independent)? Express your answer as a number with units.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, thechocolatblanc
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 21.06.2019 18:30, tae8002001
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 21.06.2019 23:00, LarryJoeseph
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1
You know the right answer?
What is the activation energy of a reaction whose rate constant doubles when the temperature is incr...
Questions in other subjects:

English, 16.06.2021 16:20

Mathematics, 16.06.2021 16:20





Physics, 16.06.2021 16:20

History, 16.06.2021 16:20


History, 16.06.2021 16:20

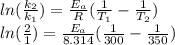
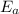 .
. kJ
kJ

