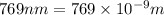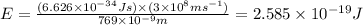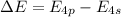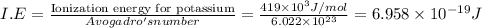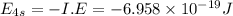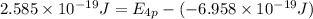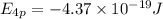Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1

You know the right answer?
The ionization energy for potassium 419 kj/mol. the wavelength of light emitted when an excited k at...
Questions in other subjects:


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01




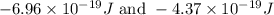
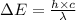
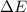 = change in energy
= change in energy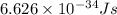
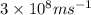
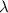 =
=