
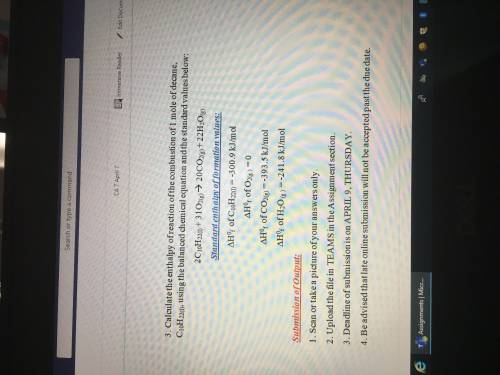
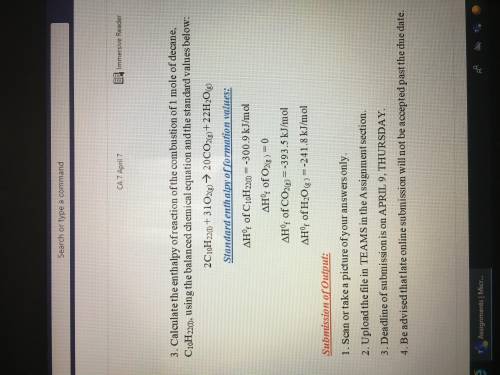
Calculate the enthalpy of the formation of butane, C4H10, using the balanced chemical equation and the standard value below:
4C(s) + 5H2(g) => C4H10(g)
Standard enthalpy of formation values:
(Delta Triangle)H^0 of C(s)= -393.5kJ/mol
(Delta triangle)H^0f of H2(g)=-285.8 kJ/mol
(Delta triangle)H^0f of C4H10(g)=-2877.6kJ/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, acaciacoats
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 23:00, lulprettyb
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 01:00, stefaniethibodeaux
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Calculate the enthalpy of the formation of butane, C4H10, using the balanced chemical equation and t...
Questions in other subjects:

Mathematics, 07.04.2021 16:30

Mathematics, 07.04.2021 16:30




World Languages, 07.04.2021 16:30


Social Studies, 07.04.2021 16:30

History, 07.04.2021 16:30

Mathematics, 07.04.2021 16:30



